Lausanne (Switzerland), Amsterdam (the Netherlands)
Debiotech and the Dutch Kidney Foundation presented today for the first time to the public the 3D model of the portable artificial kidney during the symposium organized to celebrate the 50th anniversary of the foundation. The attendees were given an explanation of the user-friendly design and the state of affairs of this special project. During this symposium, 75 years of dialysis history were covered, but also the new trends were presented. The presentation of the 3D model of the portable artificial kidney is the most recent step in dialysis innovation.Tom Oostrom, Director of the Dutch Kidney Foundation, and Frédéric Neftel, Founder and President of Debiotech, gave participants insight into the technology currently being developed for the portable artificial kidney. Using the 3D model, they explained the new compact components and the operation of the portable artificial kidney. “I am incredibly happy that the 3D model is there. We think it’s important to keep patients and professionals informed about the status of the development. Using this model, we can explain to them much more concretely how the portable artificial kidney will work in the future” commented Tom Oostrom. “We are very pleased with this close collaboration with the Foundation. Together we hope to make a significant improvement in the lives of dialysis patients” said Laurent-Dominique Piveteau, CEO of Debiotech.
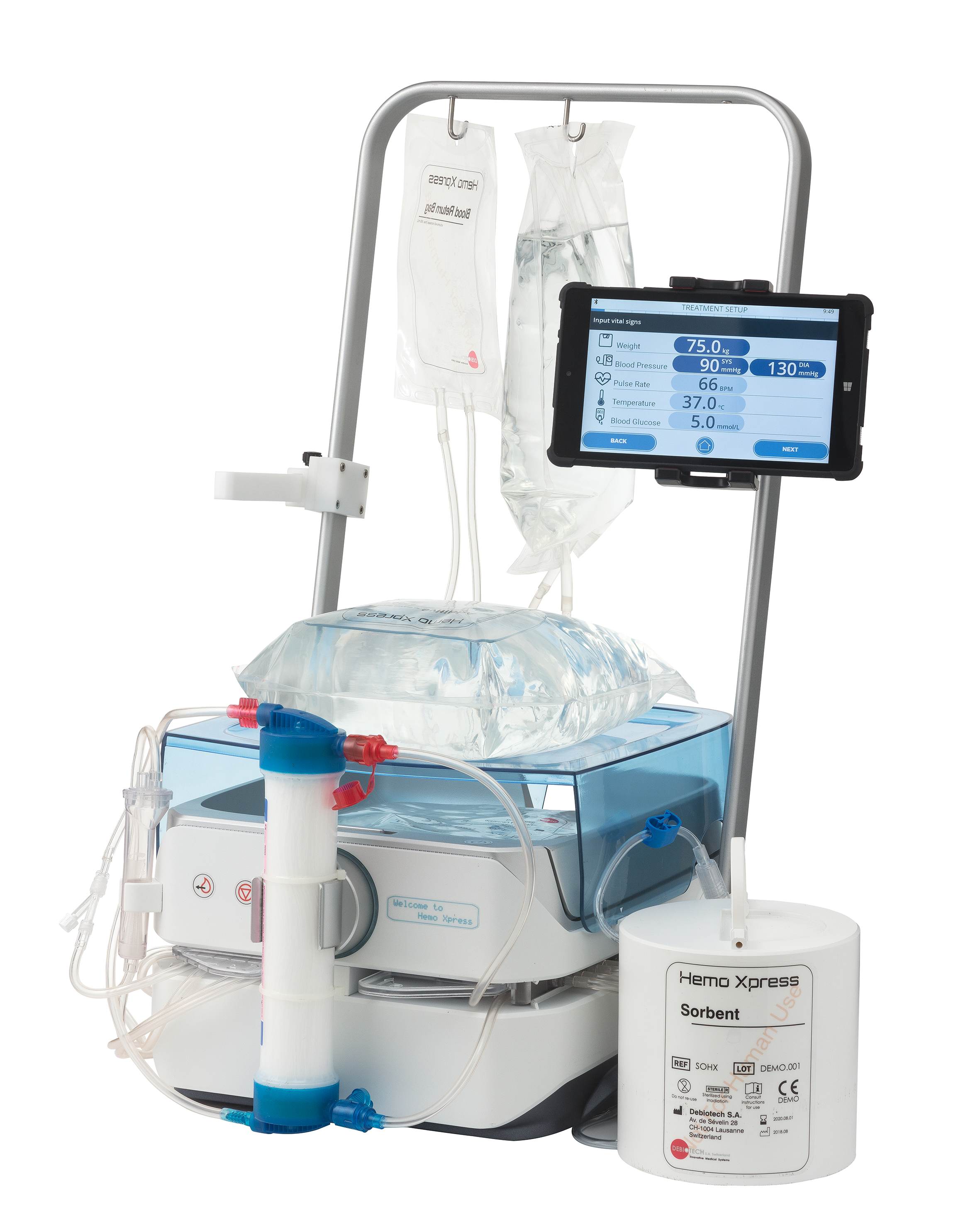
Innovating for patients
Since the invention of the first working artificial kidney in 1943 by Prof. Willem Kolff, not much has changed in hemodialysis from the perspective of patients. Dialysis patients still have to adapt their lives to their treatment instead of being able to fit their treatment into their lives. Because the market is not taking up risky innovation in dialysis treatment, the Dutch Kidney Foundation started together with Debiotech the development of the portable artificial kidney in 2014. The aim is to give patients more freedom and control over their own treatment and daily life in the future.
Where do we stand?
Together the partners are working on the further realization of the portable artificial kidney. Before clinical trials can take place, sorbent technology must be further developed and certified. After extensive testing in the lab and the first round of clinical trials with a small group of patients, the final prototype is built and clinically tested again in a group of patients. Only then can the approval process for market admission be completed. All these steps take several years and depend on the challenges that the developing parties may still encounter along the way. More information on Debiotech or the HemoXpres portable artificial kidney can be found at www.debiotech.com.

