Why mechanics is an important part of a project Unless a project’s main focus is the mechanical development itself, companies are prone to underestimating the impact of mechanics on their products.
Often, if the project is not directly related to a mechanical innovation or challenge, it may be neglected during the beginning of the project until the need arises. If it is normal to focus on the core technology that should be created, it is important, first, not to forget that all products (except 100% software ones) need a physical support. And second, not to underestimate its impact on the future product. To show it, let’s get into the different global stages of a project in medical device development through the eyes of mechanics.
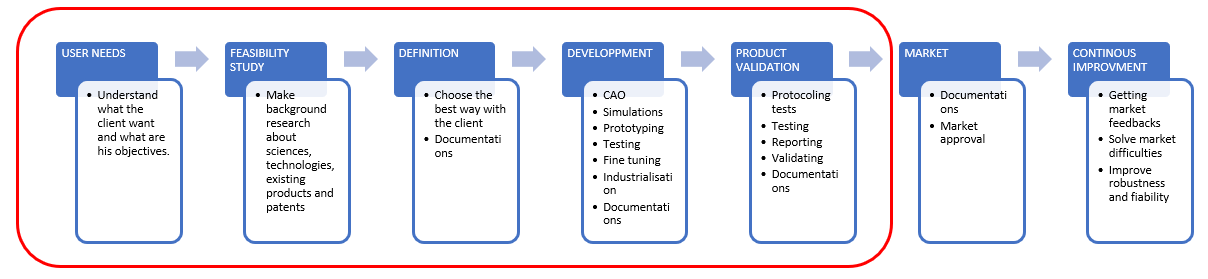
The First Step
When speaking with partners, they often forget about the size, material, form factors, fixations, how to produce and even the product esthetic. Therefore, we generally start by going into details and understanding the challenges that partners face, to get a better understanding of their needs and how they visualize their product. Having an idea is already great to begin with. Our role is to help our partners to materialize it into a successful product on the market.
Make Background Research
After clarifying the idea and defining the objectives, we proceed to the feasibility study. In this second phase, we research the technology to better understand the specificity of the product. Then, we compare it with existing products if necessary and search for patents and other limitations. It is sometimes disappointing for partners to not get their exact idea of the product. Hence, we help them to get around the limitations and push forward to find innovative solutions that could even offer a better result than originally expected.
The Development of The Mechanical Part
When the objectives are clearly defined and settled, the development phase can start. For a mechanical product, there will be several iterations of CAD, simulations, prototyping and testing to fine tune the product and tend as close as possible to an industrialized solution. And of course, all those steps must be well documented (engineering reports, design studies, etc…) as we develop a medical device. Regarding products that are not directly related to mechanics, we optimize the size, material, weight, esthetics and could even find little tricks that improve and give a little plus to them (e.g., designing ergonomic aspects of the device in close cooperation with the usability team). Obviously, it all depends on how much the partners want to invest in terms of their objectives for the target market and the time needed to realize their device. Therefore, we try to propose an optimized solution to get a reliable product with quality which considers the industrialization cost and ease of its manufacturing. The biggest work is always to choose the most suitable design. At this stage, it is important to take the time to evaluate and review the design because it will have an impact on the whole product.
Verification and Validation
After reaching the end of the development, the product is ready to enter in verification and validation, an important and unavoidable phase in the development of medical devices required by the medical regulation authorities. It is a critical time and resources consuming phase that must be well prepared. Often, startups quickly understand, through costly mistakes, the complexity and workload required to deliver the technical documentation needed for the submission to the authority. For example, it could be that closely related documents like procedures, tests and reports are not prepared beforehand and you will end up with incoherent and incomplete documentation. Another example would be that the possible sterilization effects on the material used are not anticipated. Those mistakes could lead to consequences from additional documentation to redesign of the device. With our experience, we will help and advise our partners to be more efficient and avoid these kinds of mistakes. A lot of simple tricks (from how to design the CAD files to what/how to write in the technical documentation) could be applied to have more flexible renders and intuitive mechanical documentation.
Preview
This first article in our series on mechanics in medical devices aimed to provide an overview of this important field. In future articles, we will present in more detail the development of a medical device and how mechanics is involved at each stage with, for instance, its own challenges, tips or special requirements.

