
The goal of this publication is to help you in the development of the technical documentation associated to your medical device and in its presentation. The earlier you start with a clear vision of its structure and its presentation for submission, the easier it will be to develop it efficiently without impacting drastically your development activities.
This is the first part of a series of 6 articles on this topic. It focuses on the definition of basic concepts for technical documentation management.
Targeted audience
The information gathered in this publication should be particularly useful for:
- Project Managers,
- Quality & Regulatory Managers.
Introduction
When planning to commercialize medical devices in European Union, 2 highly regulated activities are mandatory: development and maintenance of a Quality Management System (QMS) and technical documentation development. Those two activities are closely correlated as the QMS shall describe the way you plan to structure and develop your technical documentation. However, the QMS documents many other activities. The technical documentation writing is led by the project manager and validated by the quality and regulatory manager.
For submission purposes, you will have to develop a guidance/summary document allowing the reviewer to easily navigate in the set of documents you submit him/her.
In addition, if your commercialization plan aims at international reach, you must adapt your QMS and your technical documentation to multiple regulations.
What is a Quality Management System (QMS)?
The QMS is the set of documents including but not limited to the quality policy, quality manual, processes, procedures, work instructions and templates used to describe the activities of the company and the rules to generate records for the various activities of the company.
The QMS allows to document the company activities, to develop a continuous improvement approach and to demonstrate the compliance of company activities with applicable regulations.
What is a Medical Device Technical Documentation ?
The Technical Documentation is documented evidence, normally an output of the quality management system, that demonstrates compliance of a device to the Essential Principles of Safety and Performance of Medical Devices. It consists in a collection of documents prepared by the manufacturer in a clear, well-organized, readily searchable, and unambiguous manner to allow for a complete and efficient review by authorities.
What is the Summary Technical Document (STED)?
The Summary TEchnical Document (STED) is the proposed harmonized format used for regulatory submissions by the Global Harmonization Task Force (GHTF). The STED format is recognized by US and European regulators, as well as in other markets. It consists in a guidance document for the reviewer that will allow him to go step by step through your Technical Documentation.
Relationships between QMS, Technical Documentation and STED
The relationship between the three previously defined concepts is illustrated in the Figure 1.
In summary:
- The QMS shall cover the entire set of activities of your company, including technical documentation and Summary Technical Documentation writing and submissions to authorities.
- The Technical Documentation is the term used to define the entire set of documents you create while designing and developing your medical device.
- The E.P. Checklists are the Essential Principles checklists to check correct answer to the Essential Requirements established by the MDR.
- The Summary Technical Documentation is an introduction and guidance document to allow efficient review by authorities or their representatives.
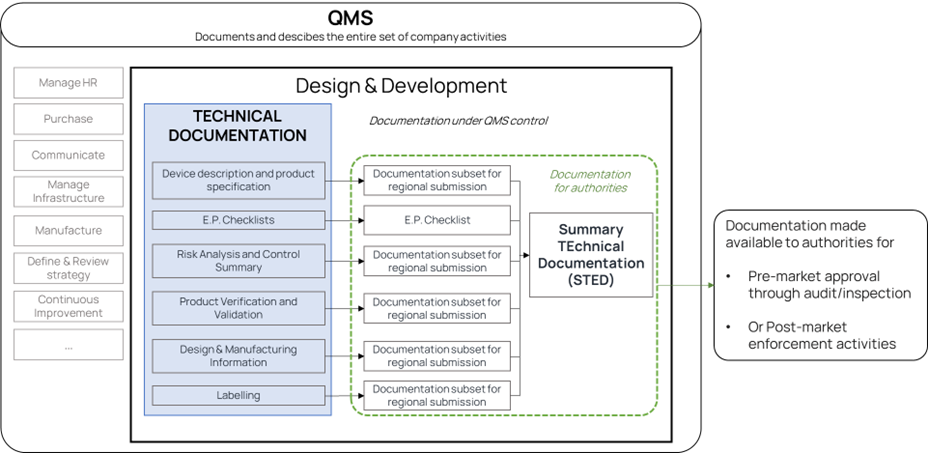
Figure 1. Relationships between QMS, Technical Documentation and STED
Authors
 |
Rémi Charrier Business Development Director r.charrier@debiotech.com |
||
|
François Cannehan |
 |
Next steps
Debiotech is glad to have the opportunity to share its knowledge with innovative companies from the MedTech industry. Your feedbacks on this publication are welcome and will be used to update it or to create new publications on topics you care about.
Continue your education on medical device development by:
- Accessing Debiotech historic publications: https://www.debiotech.com/news-grid/
- Following Debiotech on LinkedIn to be notified on new publications: https://www.linkedin.com/company/debiotech-sa
- Contacting us to ask a question or request personalized support: contact@debiotech.com
Debiotech would be proud to be your partner and support you with:
- Medical device design & development services:
- Software: Digital Health, Firmware, Embedded, SaMD
- Electronics: Design, Verification and Validation
- Mechanics: Design for micro-fabrication & fluidics systems
- Supply chain development and optimization
- Support in medical innovation management:
- Market analysis and segmentation
- IP management
- Business plan consolidation
- Partnership development


