
The goal of this publication is to help you in the development of the technical documentation associated to your medical device and in its presentation. The earlier you start with a clear vision of its structure and its presentation for submission, the easier it will be to develop it efficiently without impacting drastically your development activities.
This is the fifth part of a series of 6 articles on this topic. It focuses on the definition of the content of the summary file of the Technical Documentation.
Targeted audience
The information gathered in this publication should be particularly useful for:
- Project Managers,
- Quality & Regulatory Managers.
Technical Documentation Summary Format
Introduction
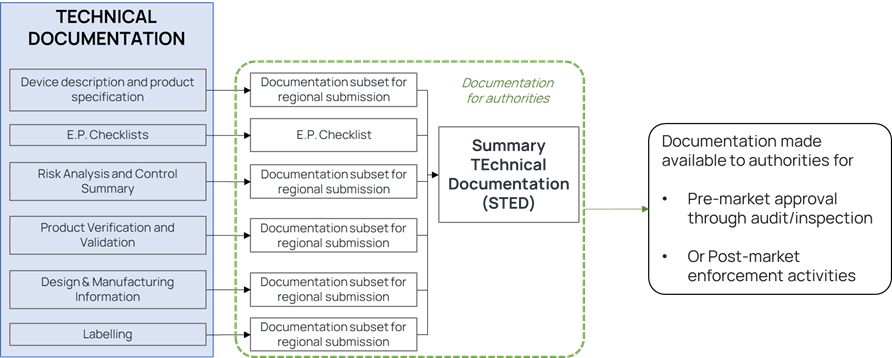
Figure 1. Illustration of STED and associated files
Without proper formatting, the most meticulously researched and prepared medical device market application can fail to pass regulatory review, costing the applicant time and money. The entire set of documentation provided by the manufacturer is usually introduced with a summary document. Once again having a clear structure for this summary document will help your reviewers. The Summary Technical Document (STED) is the proposed harmonized format used for regulatory submissions by the Global Harmonization Task Force (GHTF). The STED format is recognized by US and European regulators, as well as in other markets.
Despite this well-defined content, each project is unique and might require some adaptations. You can adapt this structure, when necessary, but it is usually a base compatible with most of the projects.
Content structure
The proposed structure for your technical documentation summary file is described in details in the STED documentation provided by the GHTF: GHTF SG1 Essential Principles of Safety and Performance of Medical Devices (STED) (imdrf.org)
A summary is provided here:
Device description & product specifications
The STED should include the following device descriptive information:
- a general description of the device including its intended use/purpose,
- the intended patient population and medical condition to be diagnosed and/or treated by the device and other considerations such as patient selection criteria,
- the principles of operation of the device,
- the Class of the device and the applicable classification rule according to GHTF/SG1/N15:2006 Principles of Medical Devices Classification etc.
- an explanation of any novel features,
- a description of the accessories, other medical devices and other products that are not medical devices, which are intended to be used in combination with the device,
- a description or complete list of the various configurations/variants of the device that will be made available,
- a general description of the key functional elements of the device, e.g., its parts/components (including software if appropriate), its formulation, its composition, its functionality. Where appropriate, this will include labelled pictorial representations of the device (e.g. diagrams, photographs, and drawings), clearly indicating key parts/components, including sufficient explanation to understand the drawings and diagrams.
- a description of the materials incorporated into key functional elements of the device and those making either direct or indirect contact with a human body.
The STED should include the following information about product specifications:
- A list of the features, dimensions and performance attributes of the medical device, its variants, and accessories (if such are within the scope of the STED), that would appear typically in the product specification made available to the end user.
- Reference to previous generation(s) or similar devices,
- Where relevant to demonstrating conformity to the Essential Principles, and to provide general background information, the STED should provide an overview of:
- the manufacturer’s previous generation(s) of the device, if such exist,
- similar devices available on the market.
Essential Principles (EP) Checklist
The STED should include an EP checklist that identifies:
- the Essential Principles of Safety and Performance,
- whether each Essential Principle applies to the device and if not, why not,
- the method used to demonstrate compliance with each Essential Principle that applies,
- the precise identity of the controlled document/s that offers evidence of compliance with each method used.
The method used to demonstrate compliance may be:
- compliance with recognized or other standards,
- compliance with a commonly accepted industry test method,
- compliance with in-house test methods,
- comparison to a similar device already available on the market.
The EP checklist should include a cross-reference to the location of such evidence both within the full technical documentation held by the manufacturer and within the STED (when such documentation is specifically required for inclusion in the Summary Technical Documentation as outlined in this guidance).
A sample checklist is included in Appendix A of the STED detailed description by the GHTF (GHTF SG1 Essential Principles of Safety and Performance of Medical Devices (STED) (imdrf.org)).
Risk analysis and control summary
The STED should summarize the risks identified during the risk analysis process and how these risks have been controlled to an acceptable level. This risk analysis should be based upon international or other recognized standards, and be appropriate to the class of the device , its complexity, and its novelty.
Product Verification and Validation
For STED documentation of product verification and validation, the level of detail will vary, and be determined by:
- the class of the device
- the complexity of the device
- the novelty of the device
As a general rule, the STED should summarize the results of verification and validation studies undertaken to demonstrate compliance of the device with the Essential Principles that apply to it. Such information would typically cover:
- engineering tests,
- laboratory tests,
- simulated use testing,
- any animal tests for demonstrating feasibility or proof of concept of the finished device,
- any published literature regarding the device or substantially similar devices.
Summary information may include:
- declaration/certificate of compliance to a recognized standard and summary of the data if no acceptance criteria are specified in the standard,
- declaration/certificate of compliance to a published standard that has not been recognized, supported by a rationale for its use, and summary of the data if no acceptance criteria are specified in the standard,
- declaration/certificate of compliance to a professional guideline, industry method, or in-house standard, supported by a rationale for its use, a description of the method used, and summary of the data in sufficient detail to allow assessment of its adequacy,
- a review of published literature regarding the device or substantially similar devices.
As a general rule, the STED should include detailed information on:
- sterilization,
- biocompatibility,
- software verification and validation,
- biological safety of devices incorporating animal or human cells, tissues or their derivatives,
- medicinal substances, if any, incorporated into the device, including compatibility of the device with the medicinal substance,
- animal studies that provide direct evidence of safety and performance of the device, especially when no clinical investigation of the device was conducted,
- clinical evidence.
These topics are not applicable to all devices.
Detailed information will describe test design, complete test or study protocols, methods of data analysis, in addition to data summaries and test conclusions.
Sterilization
Where the device is supplied sterile, the STED should contain the detailed information of the initial sterilization validation including bioburden testing, pyrogen testing, testing for sterilant residues (if applicable) and packaging validation. Evidence of the ongoing revalidation of the process shall also be provided in the form of the most recent validation report. Typically, the detailed validation information should include the method used, sterility assurance level attained, standards applied, the sterilization protocol developed against the standards, and a summary of the results against the protocol.
Biocompatibility
Details should be provided on all biocompatibility tests conducted on materials used in the device. At a minimum, tests should be conducted on samples from the finished, sterilized (when supplied sterile) device. All materials that are significantly different from materials known to be biocompatible should be characterized. Information describing the tests, the results and the analyses of data should be included.
Software Verification and Validation
The correctness of the software cannot be fully verified in the finished device. The manufacturer should provide evidence that validates the software design and development process. This information should include the results of all verification, validation and testing performed in-house and in a user’s environment prior to final release, for all the different hardware configurations identified in the labelling, as well as representative data generated from both testing environments.
Biological Safety
In the case of a medical device manufactured from or incorporating animal or human tissue or their derivative, detailed information should be provided substantiating the adequacy of the measures taken about the risks associated with transmissible agents. This will include viral clearance results for known hazards. Donor screening concerns should be fully addressed. Methods of harvesting and long-term registries should also be fully described.
Process validation results are required to substantiate that manufacturing procedures are in place to minimize biological risks.
Animal Studies
Reports of animal studies should be included, when these studies are conducted to support the probability of effectiveness in humans. These studies should be undertaken using good laboratory practices. The objectives, methodology, results, analysis, and manufacturer’s conclusions should be described. The study conclusion should address the device’s interaction with animal fluids and tissues and the functional effectiveness of the device in the experimental animal model(s). The rationale (and limitations) of selecting the animal model should be discussed.
Medicinal Substances
Yet there is no specific advice on device-medicinal combination products, but it is anticipated that the GHTF will develop recommendations in the future, at which time it will be referenced within this document.
Clinical Evidence
The STED should summarize the results of clinical evaluation studies undertaken to demonstrate compliance of the device with the Essential Principles of Safety and Performance that apply to it and should take the form of the summary Clinical Evaluation Report described in guidance published by Study Group 5 of the GHTF.
Design and Manufacturing information
Manufacturing process
The manufacturing processes for the finished device should be provided in the form of an overview of the activities and quality management system associated with the fabrication of the device. This would include design, production, assembly, final product testing and packaging of the finished medical device.
Design and manufacturing sites
If multiple facilities are involved in the design and manufacture of a device, the overview of activities for each facility should be included in the STED. If the information is identical for several sites, this should be noted. This does not include identification of sub-contractors supplying components incorporated into the device.
Labelling
The STED should contain all labelling associated with the device as described in GHTF guideline SG1/N043:2005 Labelling for Medical Devices (revised). Information on labelling will include the following subsets:
- labels on the device and its packaging,
- instructions for use, including an overview of any end-user training materials offered by the manufacturer and not included within them,
- promotional material.
Declaration of conformity
The Declaration of Conformity is not part of the STED. However, it may be annexed to the STED once the conformity assessment process has been completed. The content of the Declaration of Conformity is described in GHTF/SG1/N40:2006 Principles of Conformity Assessment for Medical Devices.
Authors
 |
Rémi Charrier Business Development Director r.charrier@debiotech.com |
||
|
François Cannehan |
 |
Next steps
Debiotech is glad to have the opportunity to share its knowledge with innovative companies from the MedTech industry. Your feedbacks on this publication are welcome and will be used to update it or to create new publications on topics you care about.
Continue your education on medical device development by:
- Accessing Debiotech historic publications: https://www.debiotech.com/news-grid/
- Following Debiotech on LinkedIn to be notified on new publications: https://www.linkedin.com/company/debiotech-sa
- Contacting us to ask a question or request personalized support: contact@debiotech.com
Debiotech would be proud to be your partner and support you with:
- Medical device design & development services:
- Software: Digital Health, Firmware, Embedded, SaMD
- Electronics: Design, Verification and Validation
- Mechanics: Design for micro-fabrication & fluidics systems
- Supply chain development and optimization
- Support in medical innovation management:
- Market analysis and segmentation
- IP management
- Business plan consolidation
- Partnership development


